Wenn Sie suchen über Clinical Evaluation Report Template – prntbl.concejomunicipaldechinu.gov.co Du hast besuchte Nach rechts Ort. Wir haben 35 Bilder etwa Clinical Evaluation Report Template – prntbl.concejomunicipaldechinu.gov.co wie Clinical Evaluation Report Template Mdr, Clinical Evaluation MDR Pack (CEP + CER) – Easy Medical Device School und auch Clinical Evaluation Report Template. Hier ist es:
Clinical Evaluation Report Template – Prntbl.concejomunicipaldechinu.gov.co

prntbl.concejomunicipaldechinu.gov.co
Clinical Development Plan Template

www.peterainsworth.com
clinical plan development template untitled document peterainsworth coe otis uky edu source portfolio
Advamed MDR IVDR Update

www.slideshare.net
mdr ivdr advamed evaluation
Clinical Evaluation MDR Pack (CEP + CER) – Easy Medical Device School

school.easymedicaldevice.com
Clinical Evaluation Report: Understanding MDD And MDR Criteria
mavenprofserv.com
Consultants For MDR Clinical Evaluation Documentation

www.i3cglobal.com
8 Key Changes To Understand In The New European MDR And IVDR

www.meddeviceonline.com
mdr ivdr key changes european understand responsibilities classification summary
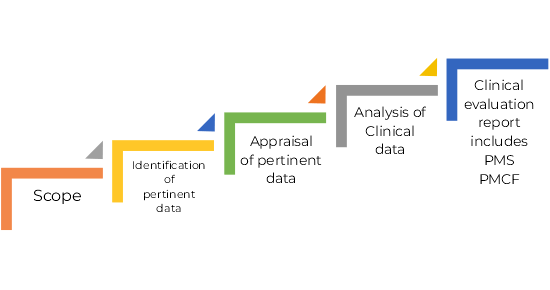
Clinical Evaluation Procedure Bundle
www.aplyon.com
procedure surveillance sop
Clinical Evaluations (MDR) | Clinical Study Templates

clinicalstudytemplates.com
How To Create A Clinical Evaluation Report (CER) Under MEDDEV & MDR
 under MEDDEV & MDR (Part 4 of 4).png#keepProtocol)
www.greenlight.guru
Best Tips: ISO 13485 Procedures With Our Free Template (Version 2016)

easymedicaldevice.com
template mdr iso medical device requirements performance safety general not after annex guidance purchase procedures tips version our shop
MDR Clinical Evaluation Report: What Is It And How To Write It?

advisera.com
clinical mdr
Clinical Evaluation Plan Template – Fill Online, Printable, Fillable

clinical-evaluation-plan-template.pdffiller.com
Clinical Evaluation Plan For Medical Devices

www.freyrsolutions.com
How To Write And Update Your EU CER | Oriel STAT A MATRIX
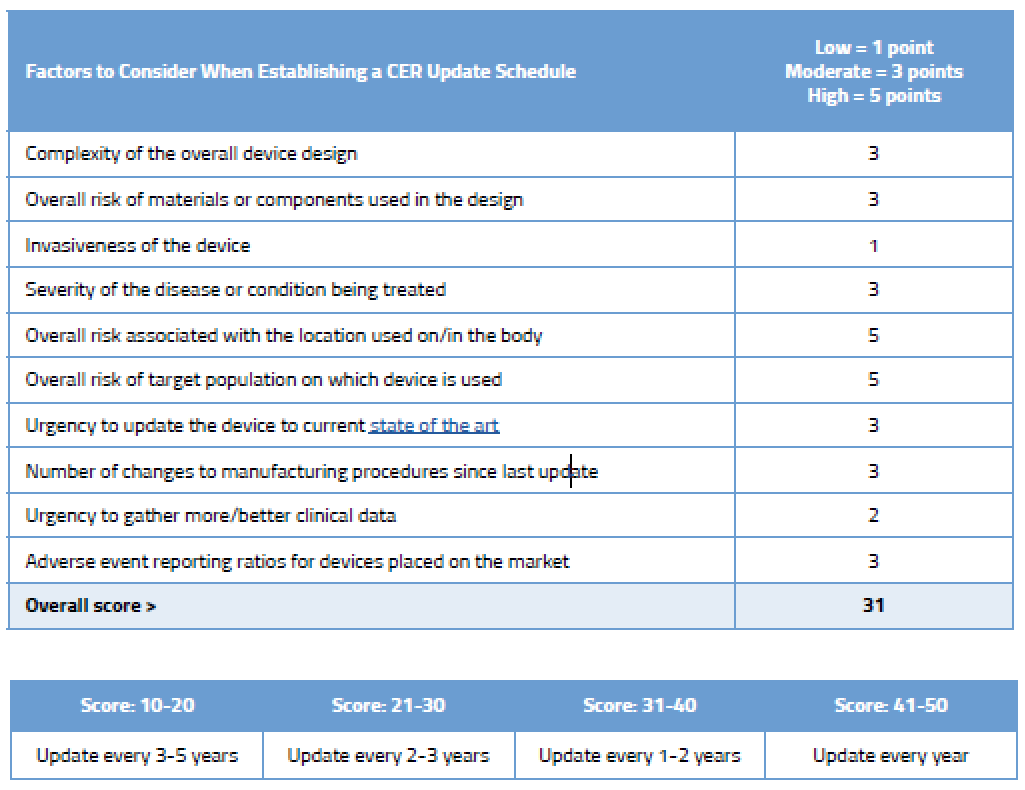
www.orielstat.com
cer often documentation
Definitive Guide To Medical Device Clinical Evaluation Reports (CER
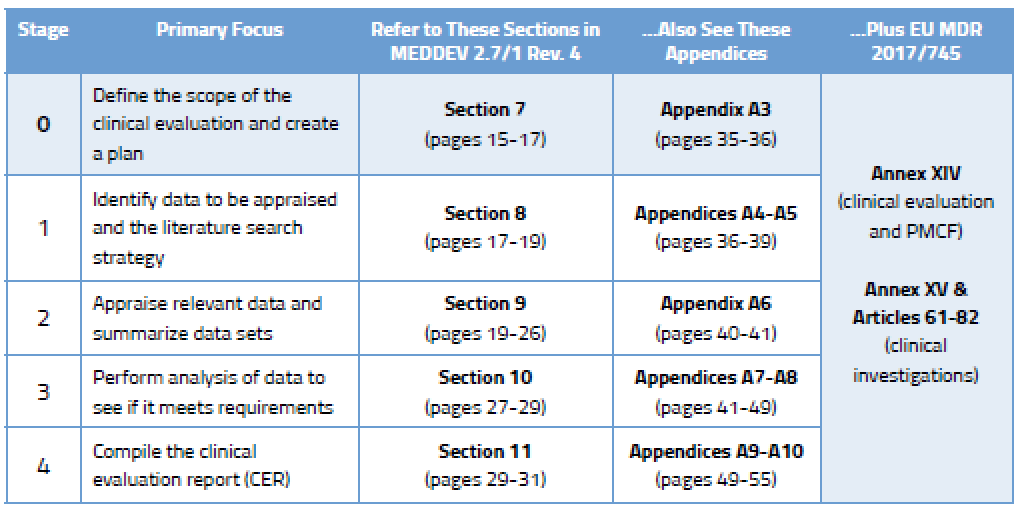
www.orielstat.com
evaluation mdr requirements cer
Clinical Evaluation Report Template – Prntbl.concejomunicipaldechinu.gov.co

prntbl.concejomunicipaldechinu.gov.co
PMS Planning And Challenges Under EU MDR – MakroCare
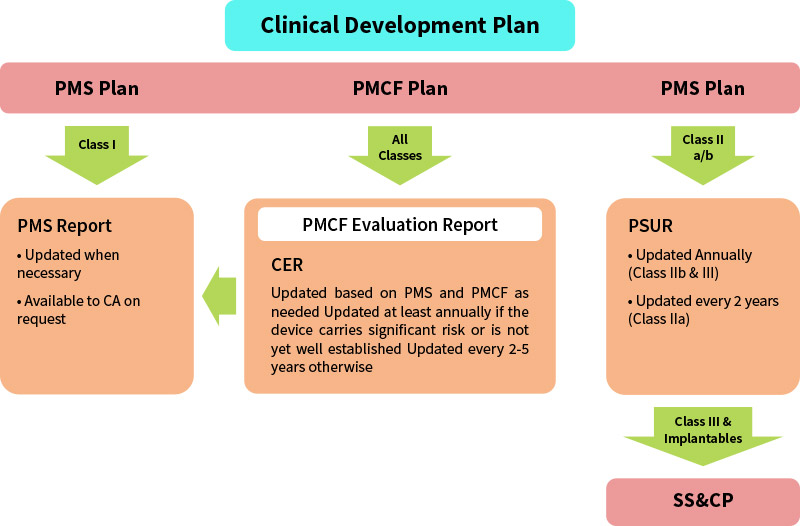
www.makrocare.com
pms mdr medical device requirements surveillance regulatory
Clinical Evaluation Plan According To EU MDR 2017/745

www.qualitymeddev.com
Clinical Evaluation SOP Plan Template [Free Download]
![Clinical Evaluation SOP Plan Template [Free Download] Clinical Evaluation SOP Plan Template [Free Download]](https://www.greenlight.guru/hs-fs/hubfs/Clinical Evaluation Procedure Template - Slide-in-covers.png?width=450&name=Clinical Evaluation Procedure Template - Slide-in-covers.png)
www.greenlight.guru
Medical Device Clinical Investigation Plan (CIP) | ISO 14155:2020 Compliant
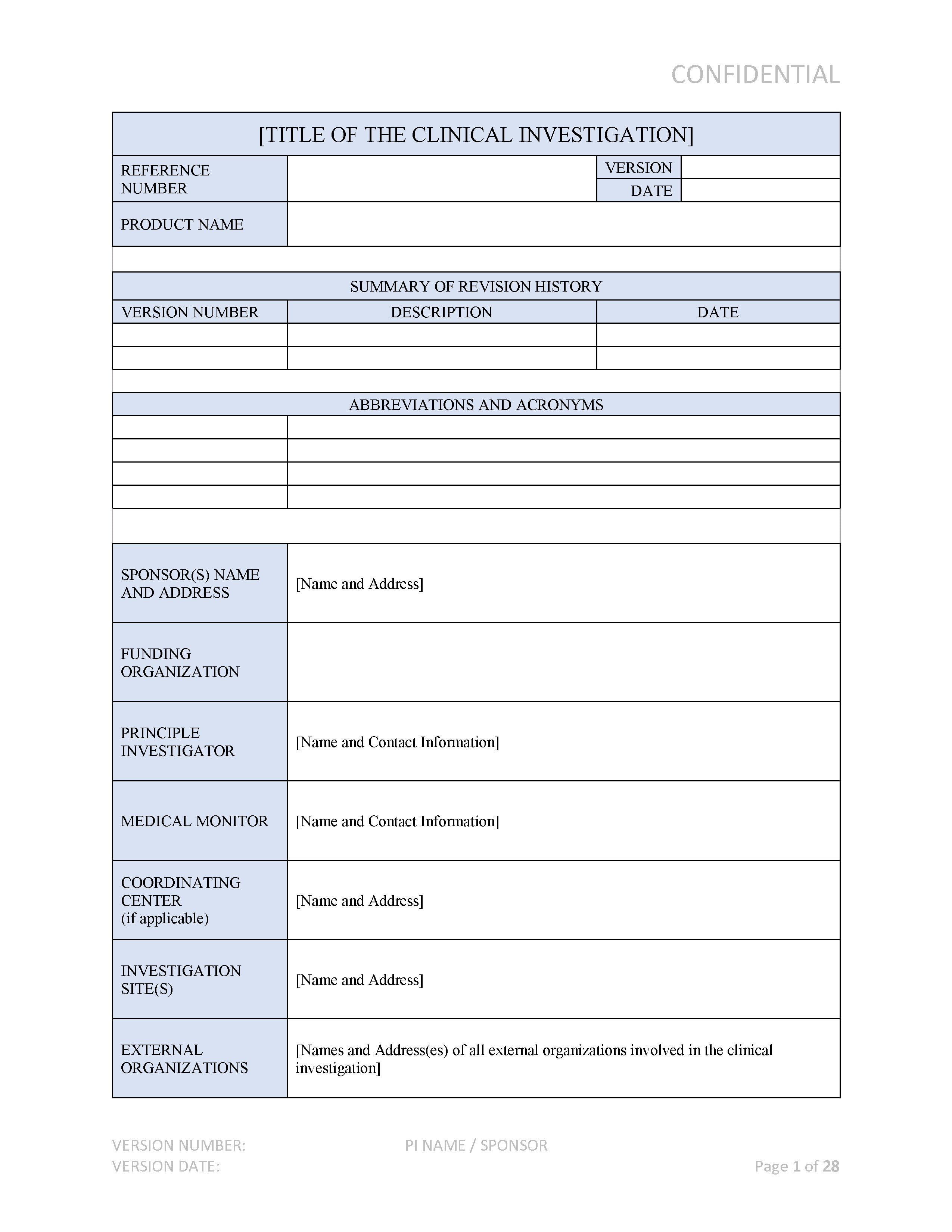
www.aplyon.com
clinical plan investigation medical device cip 2020 iso
MDR Clinical Evaluation Reports And Plans For Medical Devices
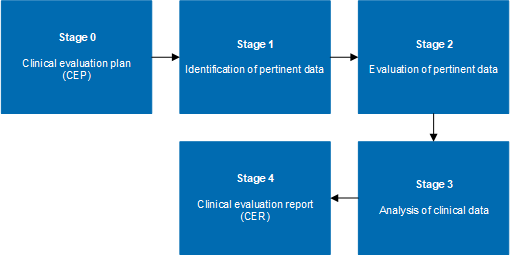
www.thinqbetter.com
stages mdr cer cep transferred stage
Clinical Evaluation Report Template Mdr

templates.rjuuc.edu.np
Clinical Evaluation Plan Template – Produce EU MDR-compliant CEPs For

www.youtube.com
Literature Review Best Practices Accelerate EU-MDR Post-Market
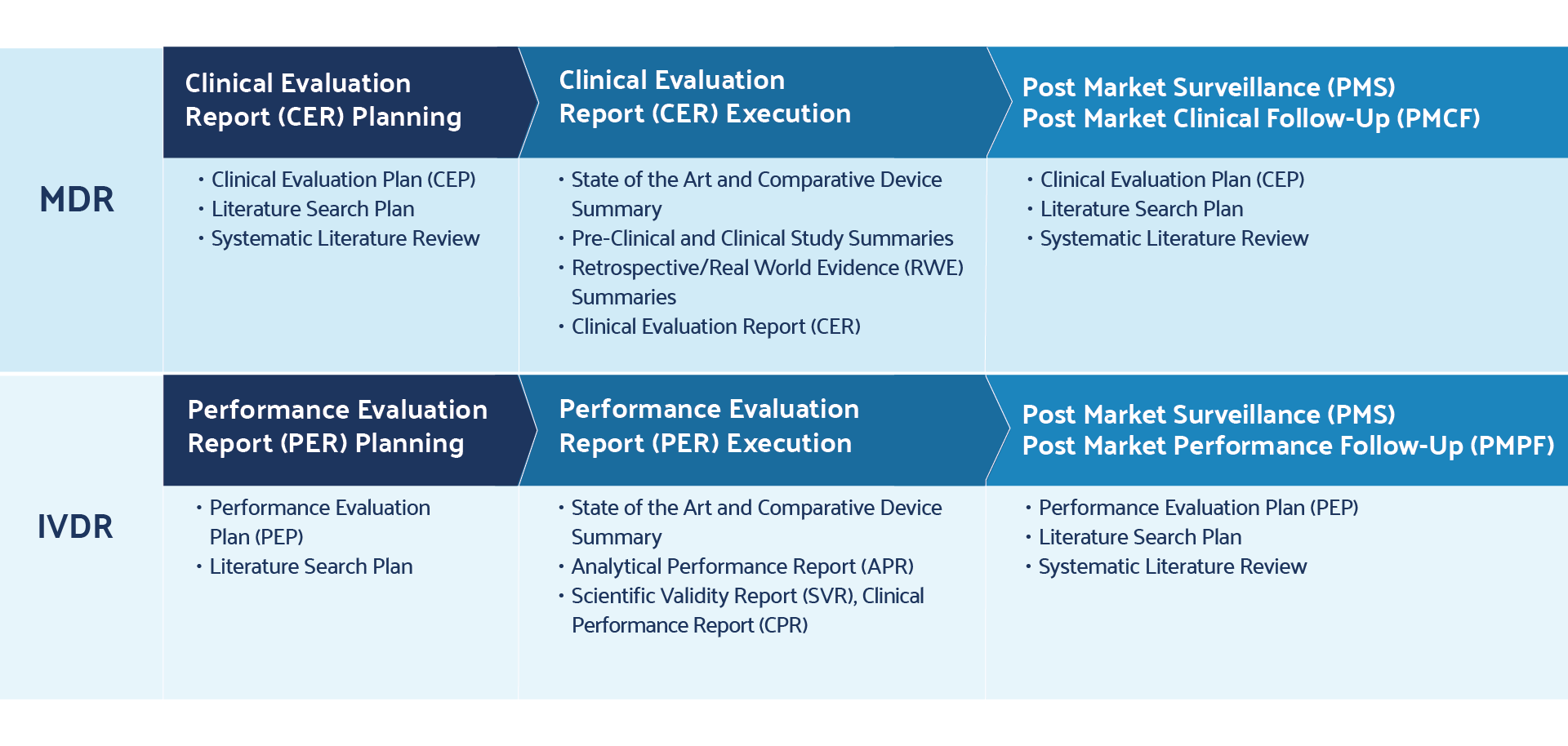
www.distillersr.com
Clinical Evaluation Plan Template
old.sermitsiaq.ag
Clinical Evaluation Report Writing For EU MDR
www.mantrasystems.co.uk
evaluation clinical report medical mdr device reports eu regulation environment fit where into writing fig
Clinical Evaluation Report Template

templates.rjuuc.edu.np
Clinical Evaluation Plan Template 2020-2022 – Fill And Sign Printable

www.uslegalforms.com
evaluation form
Clinical Evaluation Report Template

templates.hilarious.edu.np
Clinical Evaluation Report (New EU MDR Regulation) 2017/745
www.meddevicecorp.com
Claiming Equivalence Under EU MDR | Clin R

clin-r.com
FREE 15+ Sample Evaluation Reports In PDF | MS Word | Apple Pages

www.sampletemplates.com
evaluation report clinical sample reports pdf templates
Clinical Evaluation Procedure Bundle

www.aplyon.com
procedure evaluation marking
Literature Search Protocols, SOTA Reviews For MDR Compliance
www.mantrasystems.co.uk
Mdr ivdr key changes european understand responsibilities classification summary. Template mdr iso medical device requirements performance safety general not after annex guidance purchase procedures tips version our shop. Clinical evaluation mdr pack (cep + cer) – easy medical device school

