Wenn Sie suchen über New EU MDR Regulations and Revamp of the Medical Device Directive Du hast besuchte Nach rechts Ort. Wir haben 35 Bilder etwa New EU MDR Regulations and Revamp of the Medical Device Directive wie Infographic: The Medical Device Regulation | TÜV SÜD, Key Aspects of New EU Medical Devices Regulation (EU 2017/745 und auch MDR e-Book: How will the new Regulation impact your business? · MDlaw. Mehr lesen:
New EU MDR Regulations And Revamp Of The Medical Device Directive
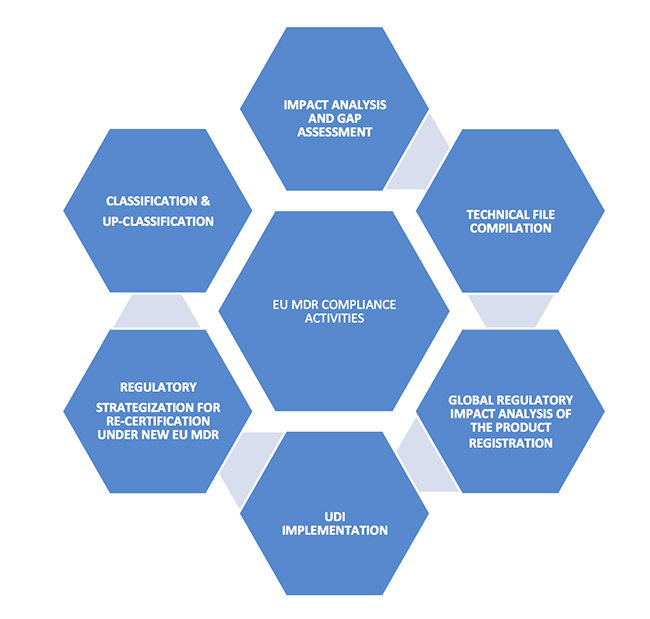
medtechintelligence.com
mdr eu device medical market compliance regulations january directive
The EU Medical Device Regulations (EU MDR 2017/745) In A Nutshell

planetinnovation.com
mdr regulations regulation regdesk nutshell fitform ivdr ec implementation warns medtech regulatory apacmed mtaa deadline complying guidance position
Infographic: The Medical Device Regulation | TÜV SÜD

www.tuvsud.com
Key Aspects Of New EU Medical Devices Regulation (EU 2017/745
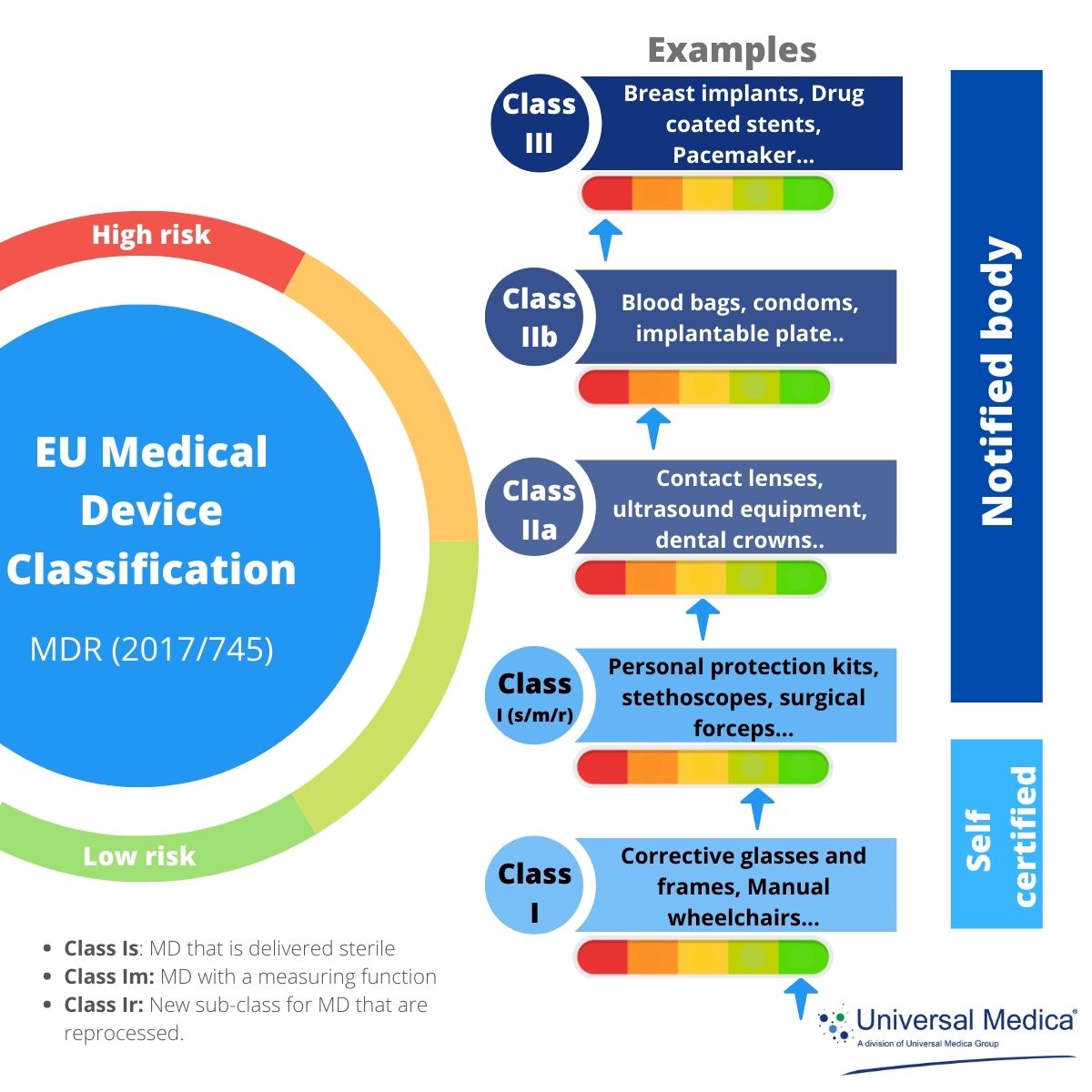
www.universalmedica.com
MDR E-Book: How Will The New Regulation Impact Your Business? · MDlaw

mdlaw.eu
mdr regulation book impact business will device medical eu
MDR 2017 745 Timeline. Implementation Of The Medical Device Regulation

www.presentationeze.com
mdr regulation 745 training validation implementation impact
EU-MDR Certification | Medical Device Regulation – IAS

www.iascertification.com
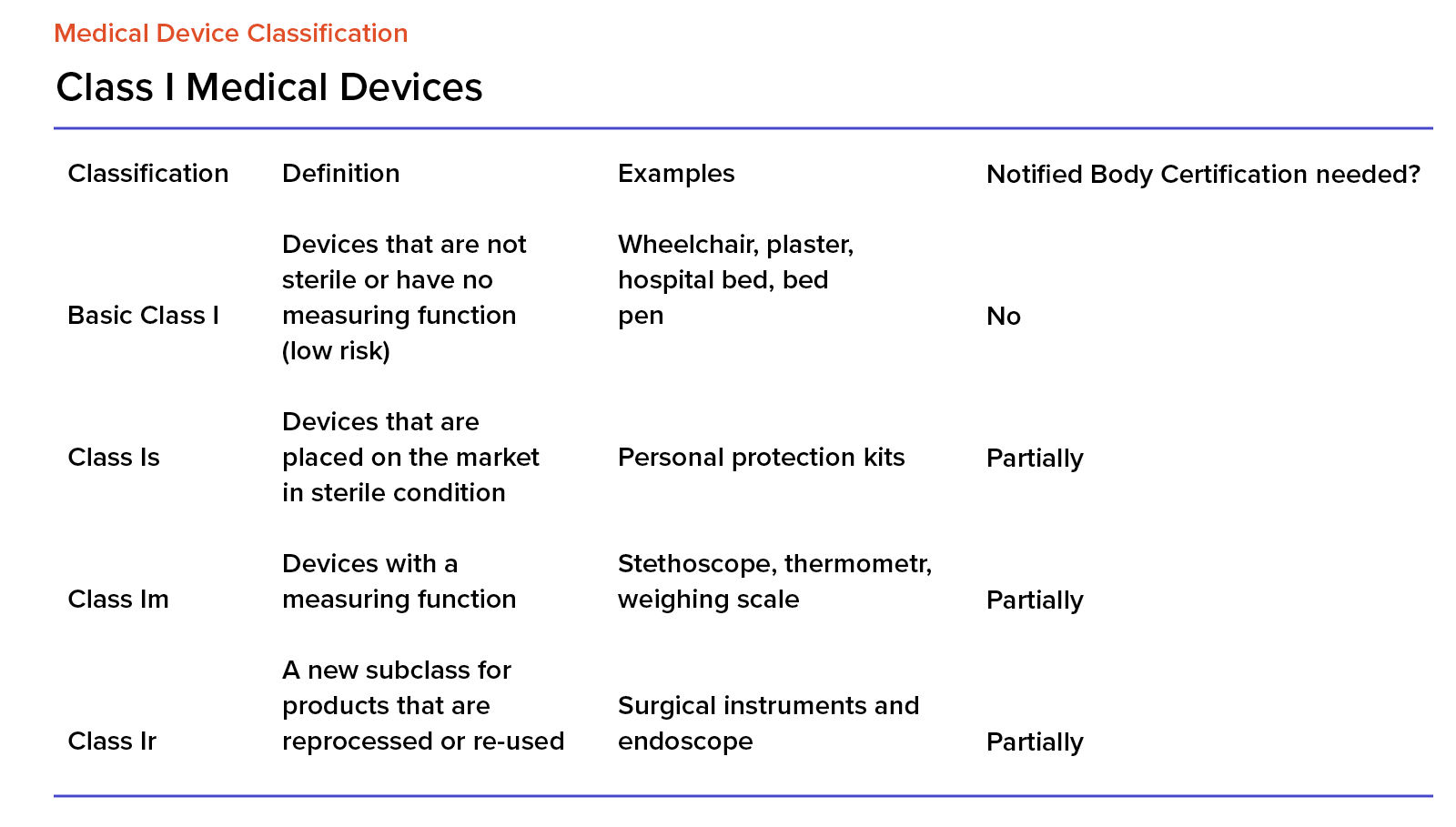
EU MDR Device Classification Guide | Advisera
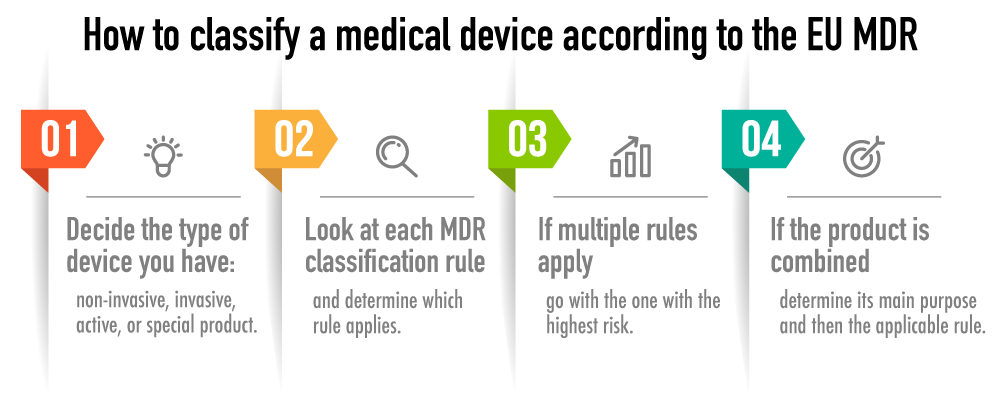
advisera.com
mdr devices rules
Are You Ready For The MDR? This Is How New EU Regulations May Impact

luzernbaar.ch
Compliance With New EU MDR In 2020 | GreenSoft Technology

www.greensofttech.com
EU MDR Implementation Guide For Medical Devices – MDCG
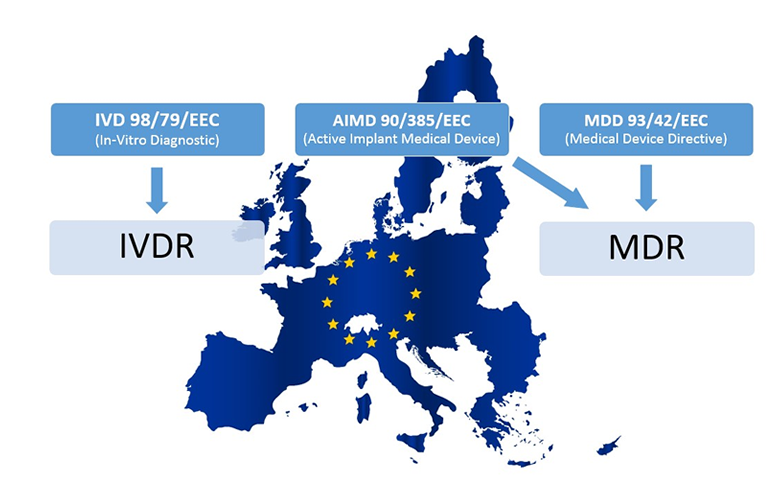
www.regulatoryglobe.com
mdr regulatory regulation devices implementation
EU MDR: Key Changes And Important Steps | Scilife
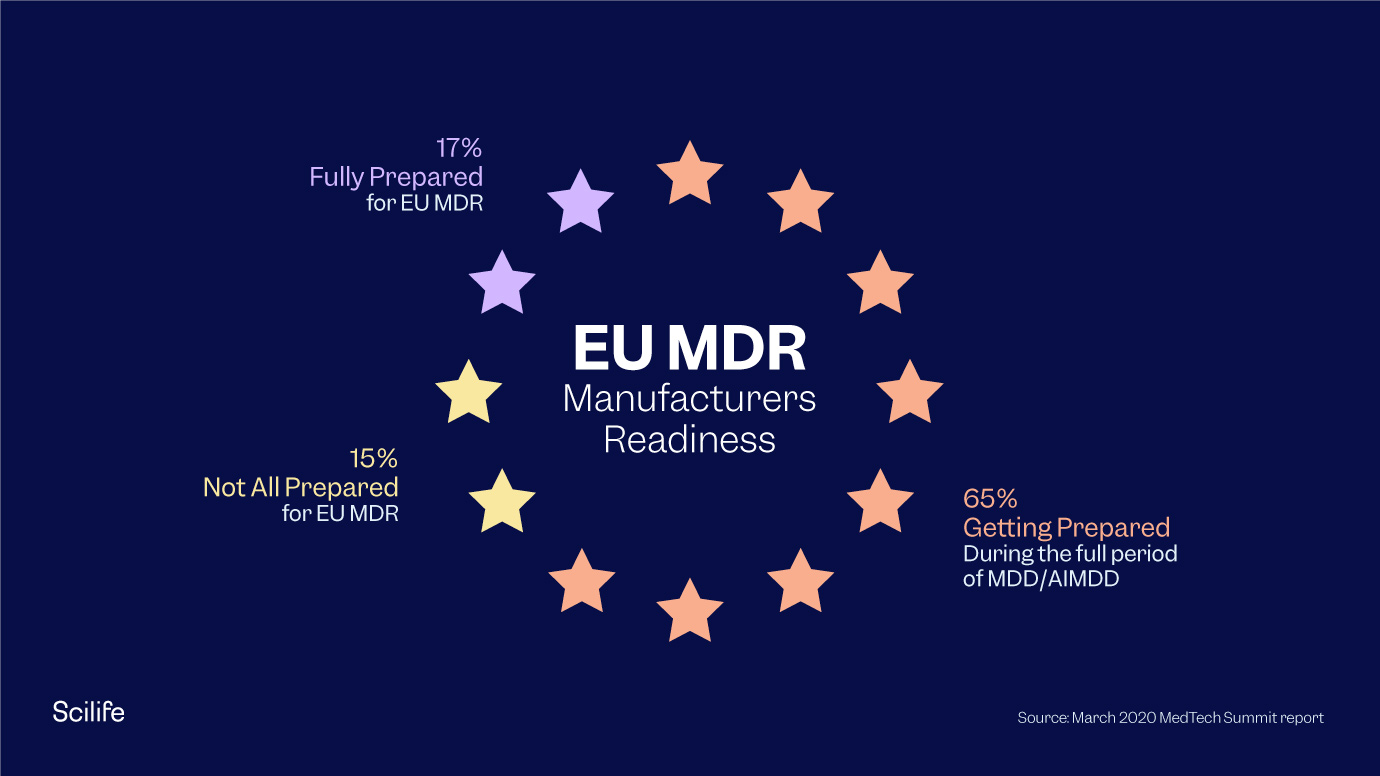
www.scilife.io
mdr changes steps regulation
First Harmonised Standards Under MDR& IVDR Are Now Available! · MDlaw
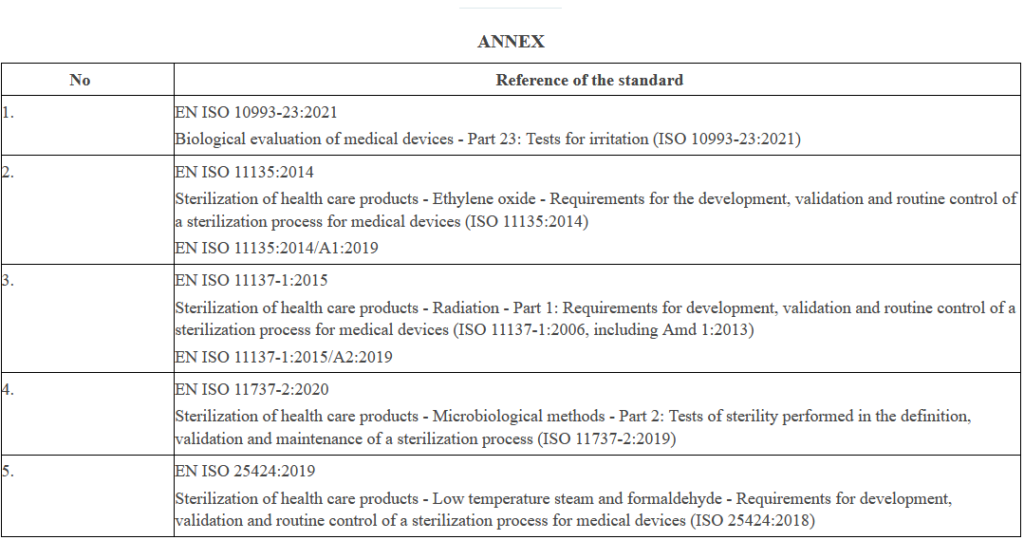
mdlaw.eu
mdr standards ivdr harmonised annex
Addressing The EU MDR And IVDR Certification Bottleneck | Blog | AssurX
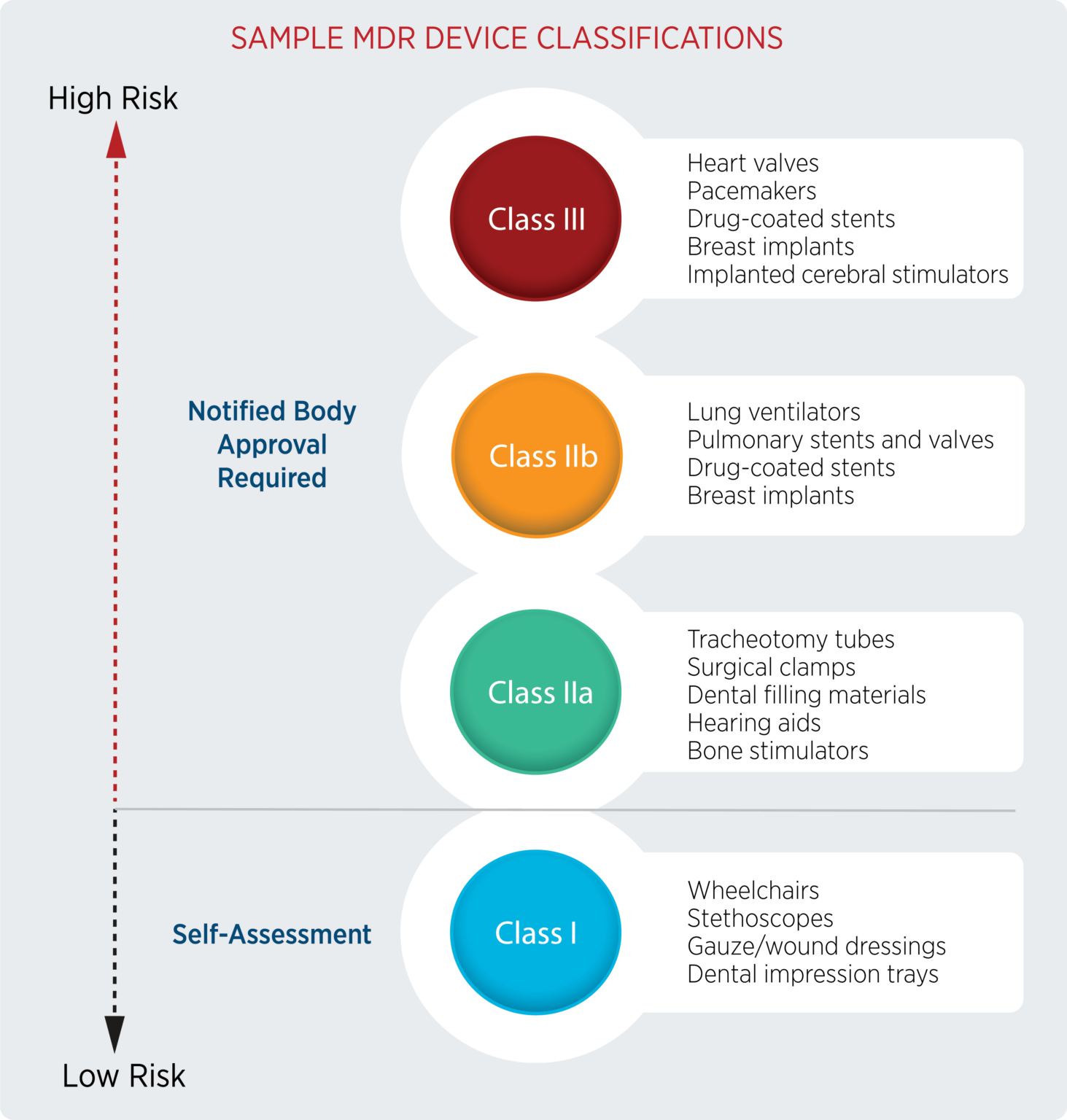
www.assurx.com
mdr ivdr addressing bottleneck prioritizing
New EU MDR Regulations And Revamp Of The Medical Device Directive
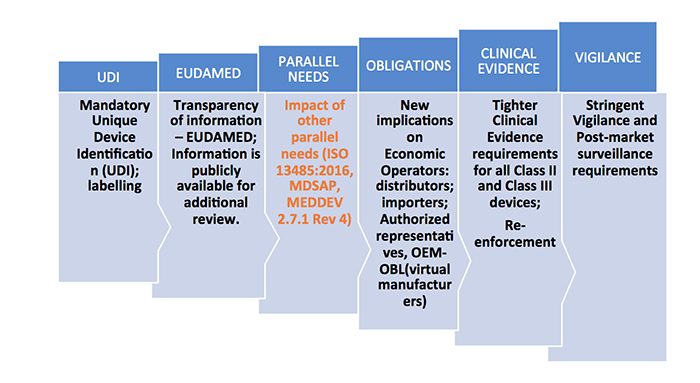
medtechintelligence.com
mdr eu medical device requirements directive safety regulations common performance specifications revamp compliance familiarization ordination terminology unique general group
EU Medical Device Regulation MDR 2017/745 | WO | TÜV Rheinland

www.tuv.com
Class 1 Medical Device Requirements | Oriel STAT A MATRIX

www.orielstat.com
class mdr eu device requirements medical devices certificate table not 2021 valid exempt mdd must manufacturer class1 manufacturers
EU Medical Device Regulation MDR (EU) 2017/745

www.ippgroupltd.com
EU MDR Compliance: Key Requirements For Medical Devices
www.acquiscompliance.com
EU MDR 2017/745 Transition Timeline [Medical Device Regulation]
![EU MDR 2017/745 Transition timeline [Medical Device Regulation] EU MDR 2017/745 Transition timeline [Medical Device Regulation]](https://easymedicaldevice.com/wp-content/uploads/2018/10/MDRTransition_update.jpg)
easymedicaldevice.com
transition mdr medical timeline device eu regulation period date easy application truth
Best Practices For A SaMD MDR Application – Rook Quality Systems

rookqs.com
EU MDR & IVDR Medical Device Labelling Requirements

www.techsollifesciences.com
EU MDR: Everything You Need To Know About Medical Device Regulation
spyro-soft.com
mdr medical device regulation documentation need
Europe Medical Devices Regulation (MDR) CE Marking Regulatory Process

www.emergobyul.cn
mdr regulatory marking regulation emergo
MDR Medical Device Regulation EU 2017 745 Timeline : PresentationEZE

www.presentationeze.com
mdr eu 745 medical device regulation timeline
Certificates Based On MDR Medical Device Regulation (EU) 2017/745 And

www.hpcosmos.com
Note For Our European Distributors In Regards To The Medical Device

somnomedics.de
mdr regulation device regards distributors requirements
MDR – Medical Devices Regulation (EU) 2017/745 | Mdi Europa

mdi-europa.com
mdr regulation medical mdi
Prepare Your Medical Device For EU MDR: 8 Trusted Resources

www.kolabtree.com
mdr
Achieve EU MDR Medical Device Compliance
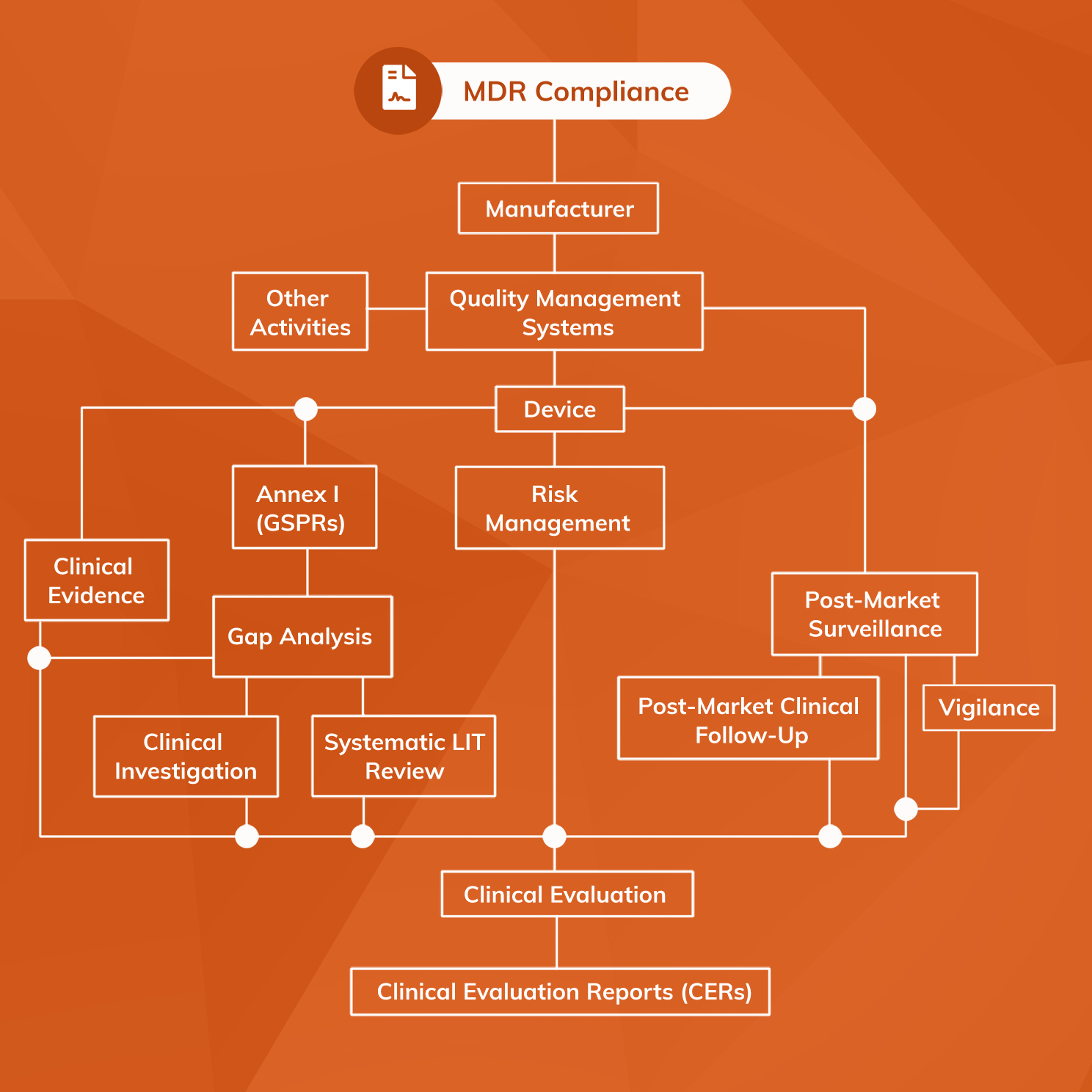
www.mantrasystems.co.uk
mdr vigilance compliance device surveillance regulation pmcf into requirements
The EU Medical Device Regulation [EU MDR] | My Language Connection
![The EU Medical Device Regulation [EU MDR] | My Language Connection The EU Medical Device Regulation [EU MDR] | My Language Connection](https://www.mylanguageconnection.com/wp-content/uploads/2020/01/EU-MDR-Timeline.png)
www.mylanguageconnection.com
mdr regulation translation affect
The Complete Guide To EU Medical Device Regulation – Spyrosoft
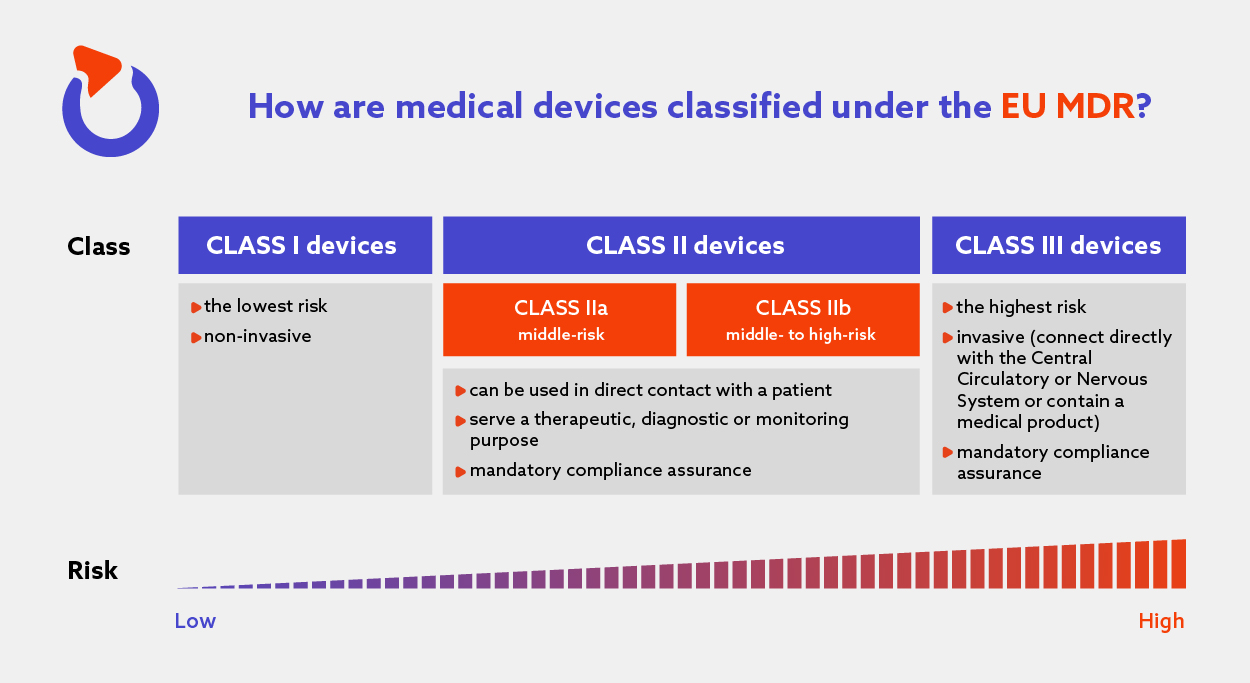
spyro-soft.com
MDR Checklist Labelling & IFU Requirements · MDlaw – Information

mdlaw.eu
mdr ifu checklist labelling requirements medical device
Medical Devices And CE Marking Process Under The EU MDR | Freyr
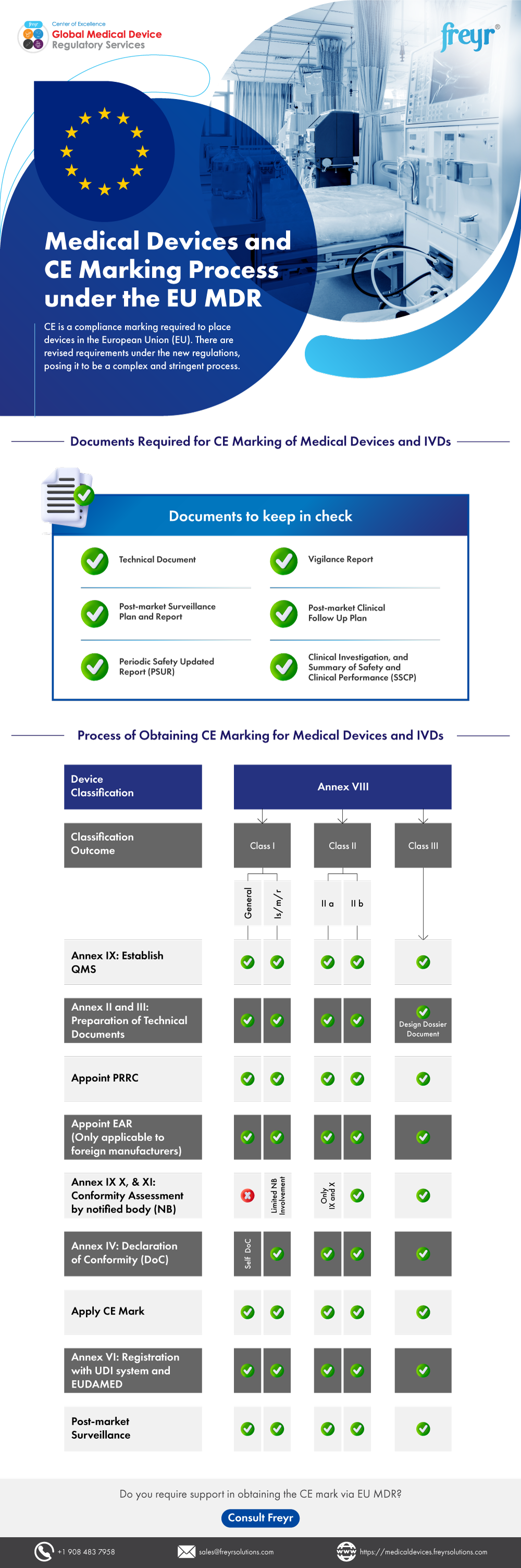
www.freyrsolutions.com
EU MDR: Update To Medical Device Regulations In Europe
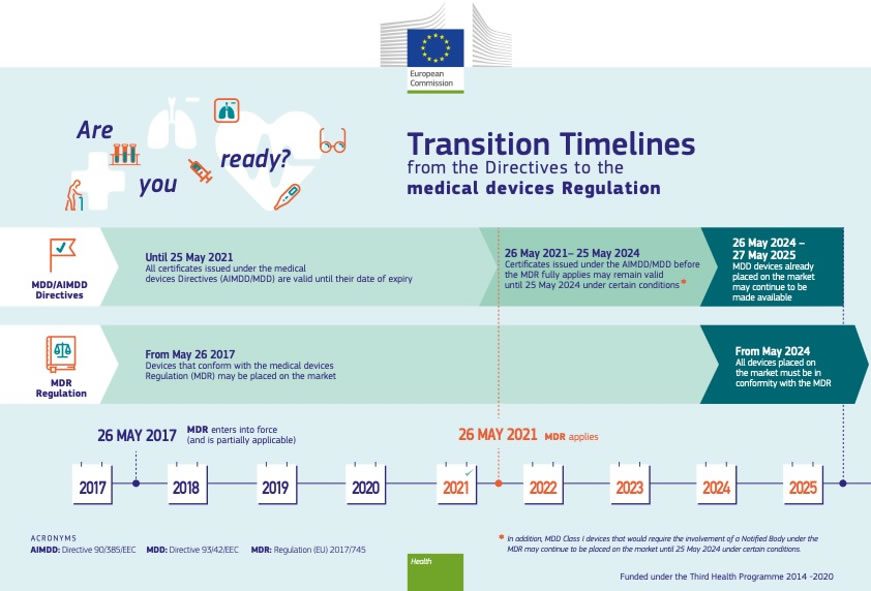
connectorsupplier.com
New eu mdr regulations and revamp of the medical device directive. Eu mdr: key changes and important steps. New eu mdr regulations and revamp of the medical device directive
