Wenn Sie suchen nach Your free guide to current MDR Classification Rules | Mi3 Du hast kam Nach rechts Ort. Wir haben 35 Fotos etwa Your free guide to current MDR Classification Rules | Mi3 wie Medical devices: EU regulations for MDR and IVDR – GOV.UK, Concept, Of, Mdr, Medical, Device, Regulation. – Pharma IT und auch Preparing For The EU MDR 2020 Changes | Oriel STAT A MATRIX. Mehr lesen:
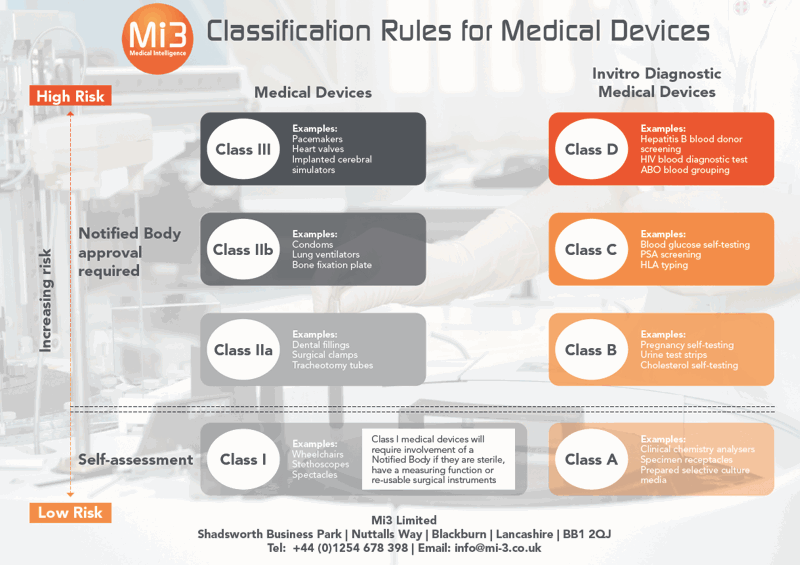
Your Free Guide To Current MDR Classification Rules | Mi3
www.mi-3.co.uk
classification mdr
Medical Device Regulation MDR Will Apply From May 26, 2021, 40% OFF
gbu-taganskij.ru
Medical Device Regulation MDR: Everything You Need To Know
blog.johner-institute.com
Regulations Updates – MDR – UK CA (2019 – 2023) » NHS Supply Chain

www.supplychain.nhs.uk
EU MDR Implementation Guide For Medical Devices – MDCG

www.regulatoryglobe.com
mdr regulatory regulation implementation
Compliance With New EU MDR In 2020 | GreenSoft Technology

www.greensofttech.com
EU MDR: EU MDR: Medical Device Regulation | TÜV SÜD

www.tuvsud.com
MDR Amendment Regulation (EU) 2023/607 – Boumans Regulatory Consulting

boumansconsulting.com
Mdr Medical Device Regulation Regulation Eu Stock Vector (Royalty Free

www.shutterstock.com
MDR Medical Device Regulation – Qu'est-ce Que Cela Signifie

www.aed.ch
mdr medical regulation signifie regulations
MDR Article 18 – Medical Device HQ

medicaldevicehq.com
Eu Mdr Declaration Of Conformity Template

flamlabelthema.netlify.app
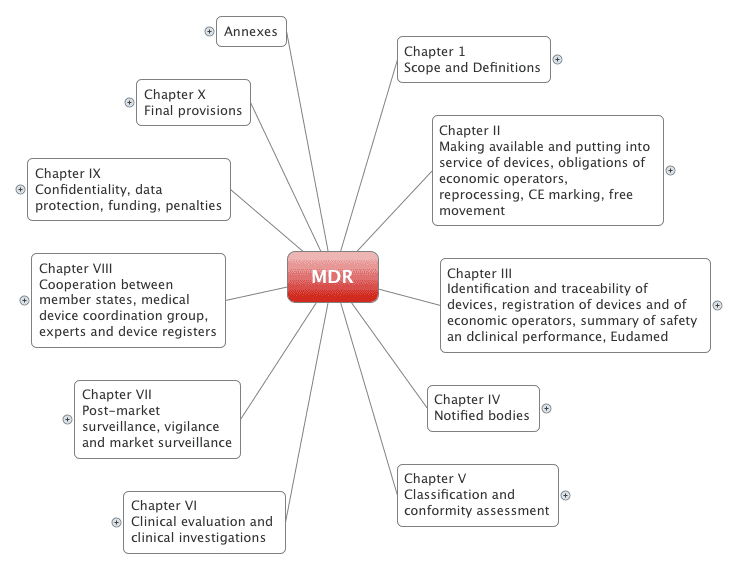
Medical Devices Regulations (MDR)

blog.cosmotrace.com
EU MDR Compliance: Key Requirements For Medical Devices
www.acquiscompliance.com
New EU MDR Regulations And Revamp Of The Medical Device Directive
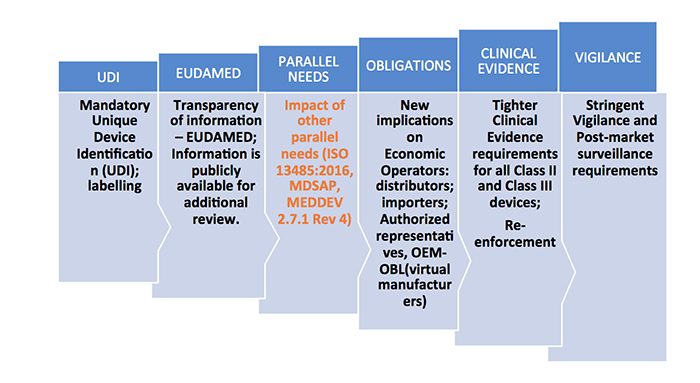
medtechintelligence.com
mdr eu medical device requirements directive safety regulations common performance specifications revamp compliance familiarization ordination terminology unique general group
MDR Classification Rules — Medical Device Regulatory Guide
+MDR+Classification+Rules.png?format=1500w)
www.mdr.guide
Infographic: The Medical Device Regulation | TÜV SÜD

www.tuvsud.com
EU Medical Device Regulation MDR (EU) 2017/745

www.ippgroupltd.com
Preparing For The EU MDR 2020 Changes | Oriel STAT A MATRIX
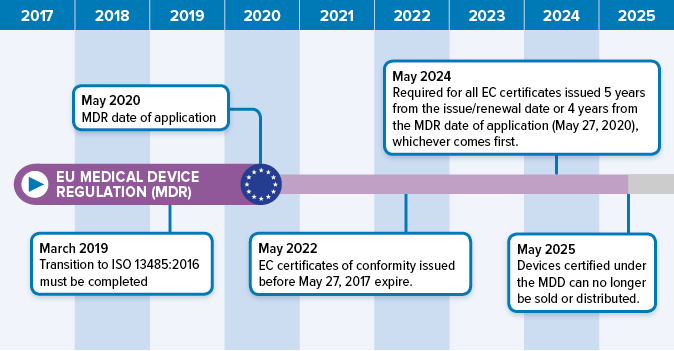
www.orielstat.com
mdr eu transition regulation timeline medical device timelines mdd changes directive difference ce overview marking compliance
Medical Device Regulations

crfweb.com
mdr regulation regulatory regulations additions impacts
6 Major Implementations In The EU Medical Devices Regulation (MDR

www.stendard.io
mdr mdd eu medical regulation major device implementations devices stricter directives enforced showing much illustration been over has
Medical Device Regulation Definition | Arena

www.arenasolutions.com
Medical Device Regulation MDR
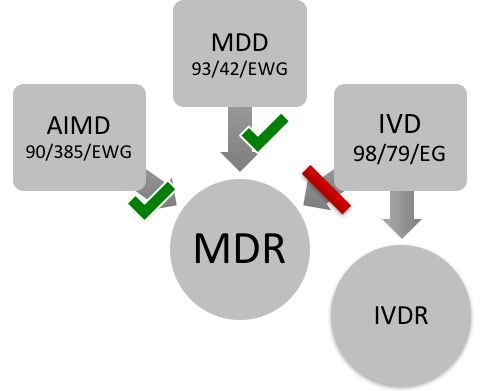
www.johner-institute.com
mdr regulation device ivdr mdd johner institut richtlinie medizinprodukte verordnung
Medical Devices: EU Regulations For MDR And IVDR – GOV.UK
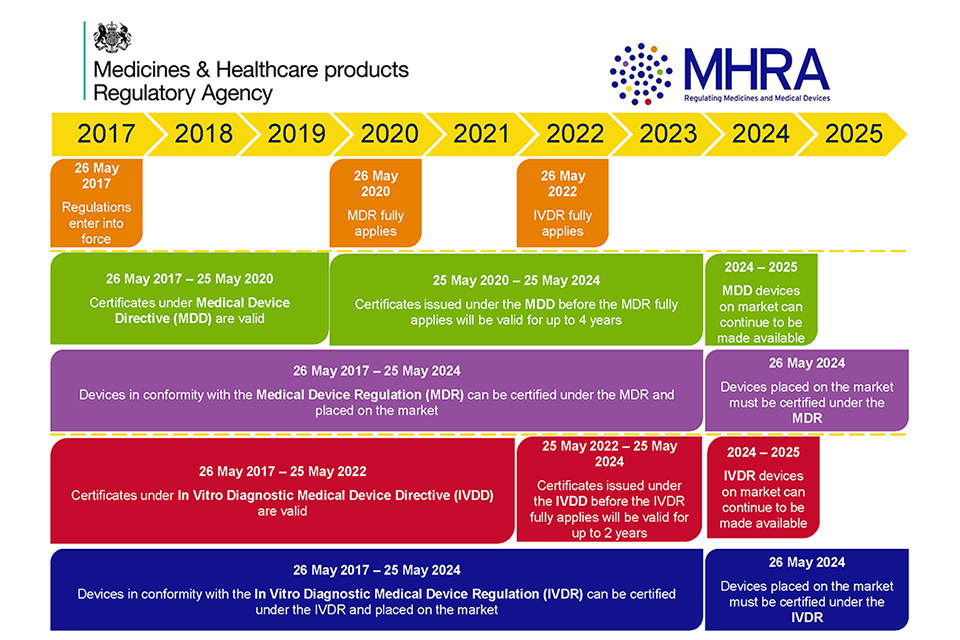
www.gov.uk
mdr ivdr medical eu devices mhra regulations device guide period gov certificate under timeline transition market ce life directives guidance
Concept, Of, Mdr, Medical, Device, Regulation. – Pharma IT

pharmait.dk
EU Regulation: Transitioning From The MDD To MDR
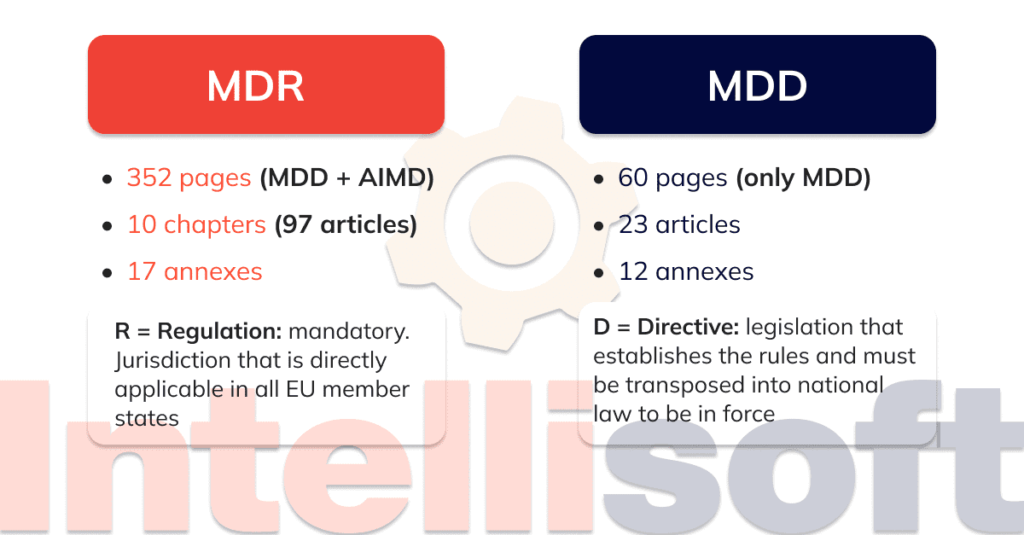
intellisoft.io
MDR Guidance | Medical Device Regulatory Guide

www.mdr.guide
What You Need To Know About The MDR Classification Rules
www.avanti-europe.ch
mdr classification rules europe under
Medical Device Regulation MDR Will Apply From May 26, 2021, 56% OFF
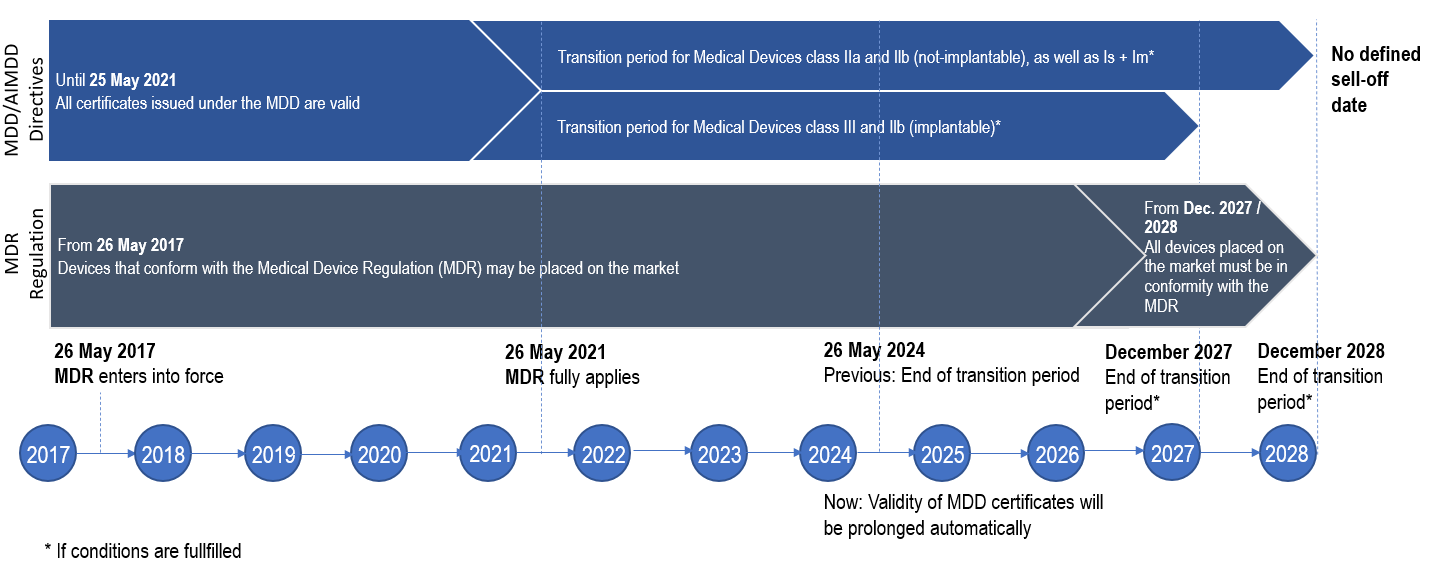
gbu-taganskij.ru
Class 1 Medical Device Requirements | Oriel STAT A MATRIX
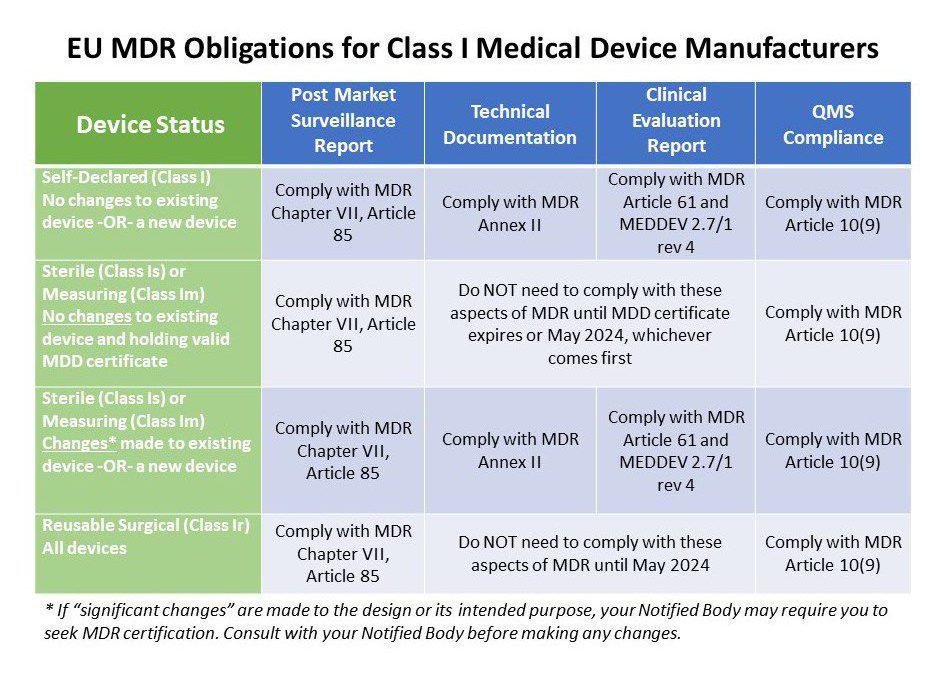
www.orielstat.com
mdr eu requirements class devices medical device table surveillance manufacturers market post 2021 manufacturer applicable
Medical Device Regulation MDR 2017/745 – Course And Certificate

gxp-training.com
EU Finalizes New Medical Device Regulations (MDR) Which Update The

www.fastrial.com
mdr device medical changes ivdr market eu europe devices regulatory framework largest deep second world unanimously council introducing adopts european
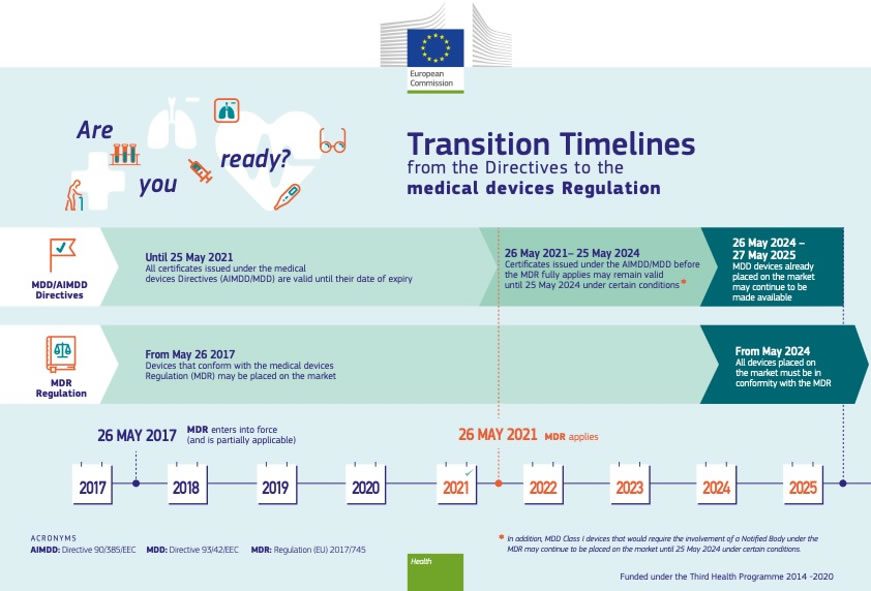
EU MDR 2017/745 Transition Timeline [Medical Device Regulation]
![EU MDR 2017/745 Transition timeline [Medical Device Regulation] EU MDR 2017/745 Transition timeline [Medical Device Regulation]](https://i2.wp.com/easymedicaldevice.com/wp-content/uploads/2018/10/MedDevRegTrans.png?fit=750,679&ssl=1)
easymedicaldevice.com
mdr medical device transition eu regulation mdd timeline clarity regulations easy reduction provide companies some will
New EU MDR Regulations And Revamp Of The Medical Device Directive
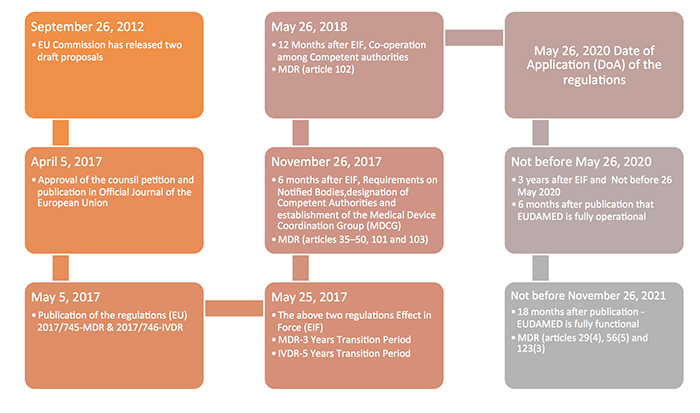
medtechintelligence.com
medical device eu directive mdr regulations revamp classification rules transition medtech timelines annexes chronology adoption events
New EU MDR Regulations And Revamp Of The Medical Device Directive
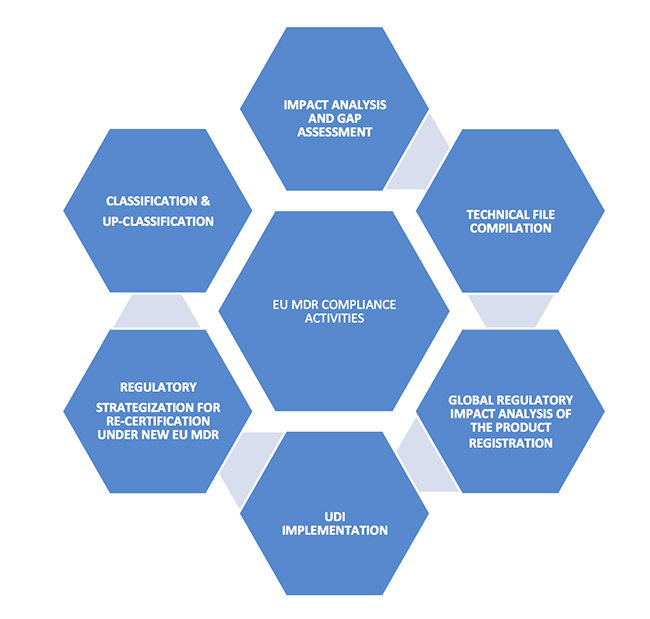
medtechintelligence.com
mdr eu device medical market compliance regulations january directive
Regulations updates. Eu mdr compliance: key requirements for medical devices. Medical device regulation mdr: everything you need to know

